In-Depth Guide to Endothermic Reactions and Their Real-Life Applications
Have you ever wondered how ice packs magically absorb heat and cool injuries in seconds? This remarkable phenomenon occurs through an endothermic reaction, where heat energy is absorbed, leading to a cooling effect.
Endothermic reactions are essential in both nature and technology, influencing everything from photosynthesis to the refrigeration systems in our homes. So, what are the critical details you need to know about these intriguing chemical processes?
In this in-depth guide, we’ll explore the definition of endothermic reactions and provide relatable examples, such as the melting of ice and the use of chemical cold packs. We’ll also compare these reactions to their exothermic counterparts and discuss their significant applications in everyday life. Prepare to uncover the fascinating world of endothermic processes and their vital role in various chemical interactions!
Introduction to Endothermic Reactions
Endothermic reactions are fascinating chemical processes that absorb heat energy from their surroundings. This heat absorption results in a cooling effect, which is crucial in many everyday applications—from making ice to supporting life through biological processes like photosynthesis. When a substance undergoes an endothermic reaction, it typically experiences a phase change, such as melting or evaporation.
For instance, consider the melting of ice into liquid water. This transformation requires energy input to break the bonds holding the ice molecules together, illustrating the principle of endothermic processes.
These reactions are not just limited to simple physical changes; they play a significant role in various industries, including cooling systems and temperature control. Understanding endothermic reactions allows us to appreciate their importance and encourages further exploration of the dynamic world of chemistry.
Definition and Basic Principles of Endothermic Reactions
Endothermic reactions are chemical processes that absorb heat energy from their surroundings. This absorption leads to a decrease in the surrounding temperature, making these reactions critical in various applications. In essence, the energy required for the reaction to occur exceeds the energy released, resulting in a net gain of energy.
Core Principles
1. Heat Absorption: During an endothermic reaction, heat is taken in, causing the reactants to undergo a transformation. For example, when ammonium nitrate dissolves in water, it absorbs thermal energy, resulting in a cooling effect.
2. Energy Transformation: Endothermic processes also involve the breaking of chemical bonds, which requires energy input. This energy can be visualized in a simple energy level diagram, where the reactants’ energy increases as they form products.
3. Examples in Nature: Photosynthesis is a quintessential example of an endothermic reaction, where plants absorb solar energy to convert carbon dioxide and water into glucose and oxygen.
Understanding these core principles emphasizes the role of endothermic reactions in both natural and industrial processes, showcasing their importance in everyday life and scientific applications.
Common Examples of Endothermic Reactions
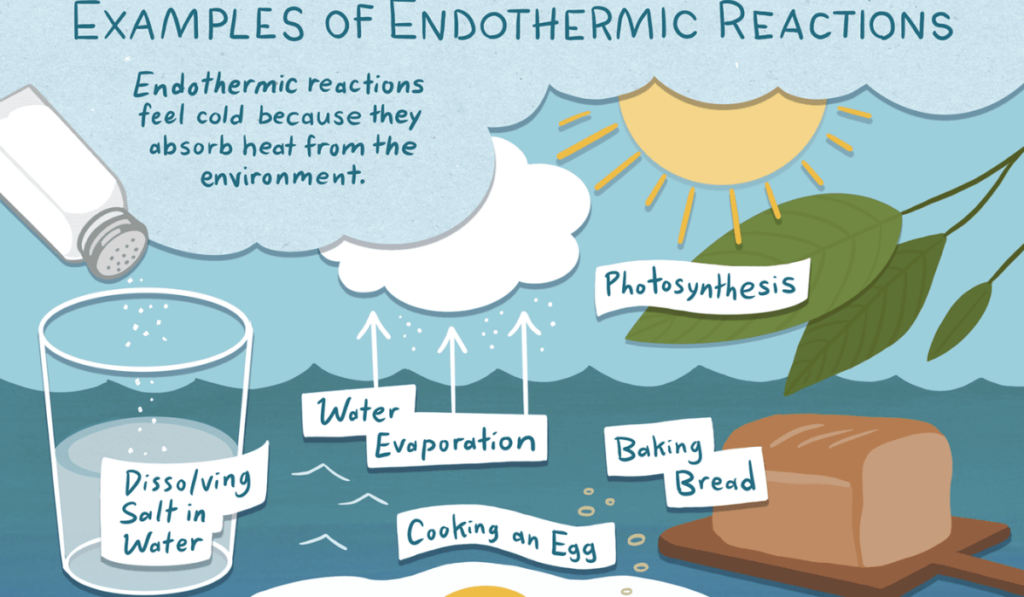
Endothermic reactions occur when a system absorbs heat from its surroundings. These reactions are essential to various processes in nature and technology. Here are some relatable examples:
Melting of Ice to Form Water
The melting of ice is a classic example of an endothermic reaction. When solid ice absorbs heat from the environment, it transitions into liquid water. This process requires heat energy to break the hydrogen bonds between water molecules, demonstrating the concept of energy absorption.
Evaporation of Liquid Water to Vapor
Evaporation is another endothermic process where liquid water transforms into vapor. As liquid water heats up, it absorbs thermal energy, causing its molecules to move more rapidly and escape into the air. This absorption of heat is why puddles disappear on hot days, showing the cooling effect of evaporation in action.
Sublimation of Dry Ice
Dry ice, which is solid carbon dioxide, undergoes sublimation when it absorbs heat from its surroundings to become carbon dioxide gas. This process is used in various applications, such as creating fog effects in theater productions. The striking visual effects come from the rapid phase change, illustrating how endothermic processes can lead to significant temperature changes and physical transformations.
Photosynthesis
Photosynthesis in plants is a vital endothermic reaction. During this process, plants absorb sunlight (thermal energy) to convert carbon dioxide and water into glucose and oxygen. This reaction is crucial for life on Earth, providing not just energy for plants but also oxygen for other organisms.
Ammonium Nitrate in Cold Packs
Chemical cold packs are practical examples of endothermic reactions in everyday life. When ammonium nitrate dissolves in water, it absorbs heat, producing a cooling effect. This is beneficial for treating injuries, as it lowers the temperature of the skin and surrounding tissues.
These examples clearly illustrate how endothermic reactions play an important role in both nature and human-made processes, highlighting their relevance in our daily lives.
Melting of ice to form water
The melting of ice to form water is a classic example of an endothermic reaction. This physical change occurs when ice absorbs heat energy from its surroundings, causing the ice to transition from solid to liquid. The temperature at which this happens is 0°C (32°F). During this process, the energy input breaks the bonds between water molecules, promoting a structural change. Notably, this cooling effect is significant in nature, such as in snow melting, and is also harnessed in chemical cold packs used for injuries, demonstrating the practical applications of endothermic processes in everyday life.
Evaporation of liquid water to vapor
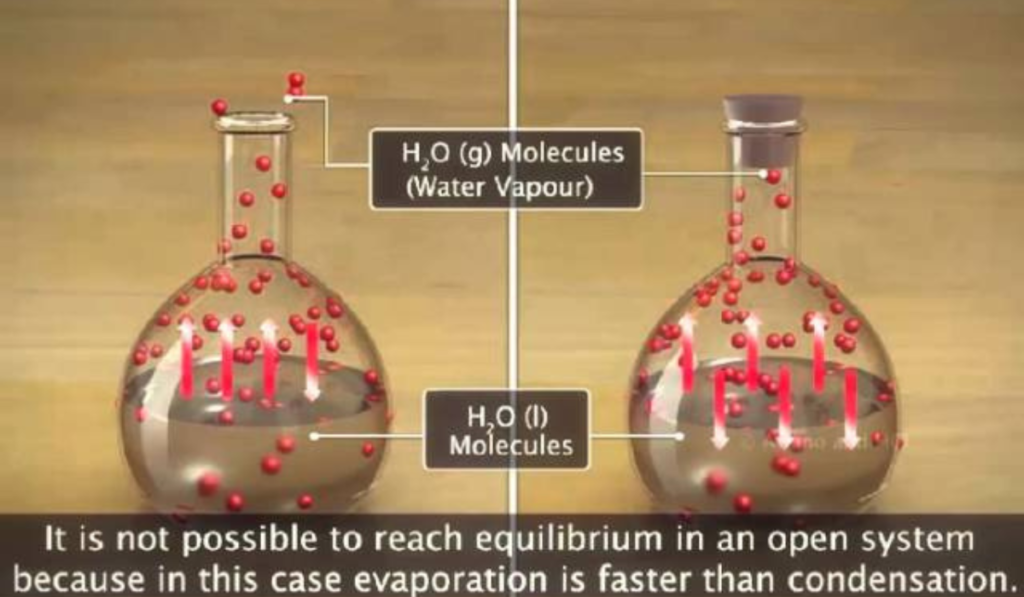
The evaporation of liquid water to vapor is a classic example of an endothermic reaction. In this process, heat energy from the surroundings is absorbed as water molecules transition from a liquid to a gaseous state. This phenomenon occurs when the molecules gain enough energy to overcome intermolecular forces. For instance, on a sunny day, water in puddles or lakes evaporates into vapor, leading to a cooling effect in the environment. This energy transformation plays a crucial role in the water cycle, affecting weather patterns, and it is vital for biological processes such as photosynthesis.
Sublimation of dry ice
Sublimation of dry ice is an intriguing endothermic reaction. When dry ice (solid carbon dioxide) is exposed to room temperature, it absorbs heat energy from its surroundings. This causes it to change directly from a solid to a gas, skipping the liquid phase. This phase change not only displays a cooling effect but also introduces practical applications, such as in refrigeration and preservation. For instance, dry ice is often used in food shipping to keep items cold without the mess of melting ice. Understanding the energy transformation during sublimation highlights the important role of endothermic processes in our everyday life.
Applications of Endothermic Reactions
Endothermic reactions are integral to various industries, highlighting their significance in our everyday life. Below are some notable applications:
1. Chemical Manufacturing
In the chemical industry, endothermic processes are essential for synthesizing various compounds. One prominent example is the use of ammonium nitrate in cold packs. When ammonium nitrate dissolves in water, it absorbs heat, creating a cooling effect beneficial for treating sports-related injuries.
2. Refrigeration Systems
Endothermic reactions play a critical role in refrigeration cycles. Refrigerants undergo phase changes that absorb heat from their surroundings, cooling the internal environment of refrigerators and air conditioners. For instance, the gas-to-liquid transition of refrigerants in cooling systems relies on their ability to absorb heat, making it efficient for temperature control.
3. Photosynthesis
Nature also showcases endothermic reactions. During photosynthesis, plants absorb sunlight (a form of energy) to transform carbon dioxide and water into glucose and oxygen. This process is vital for plant growth and contributes to the energy cycle in our ecosystem.
4. Climate Implications
The understanding of endothermic reactions aids in addressing climate concerns. Endothermic processes in the water cycle, like evaporation, play a crucial role in regulating temperatures. Recognizing their impact helps in devising sustainable solutions for environmental challenges.
In summary, these applications emphasize the importance of endothermic reactions across various fields, from medicine to environmental science, reinforcing their role in our daily lives.
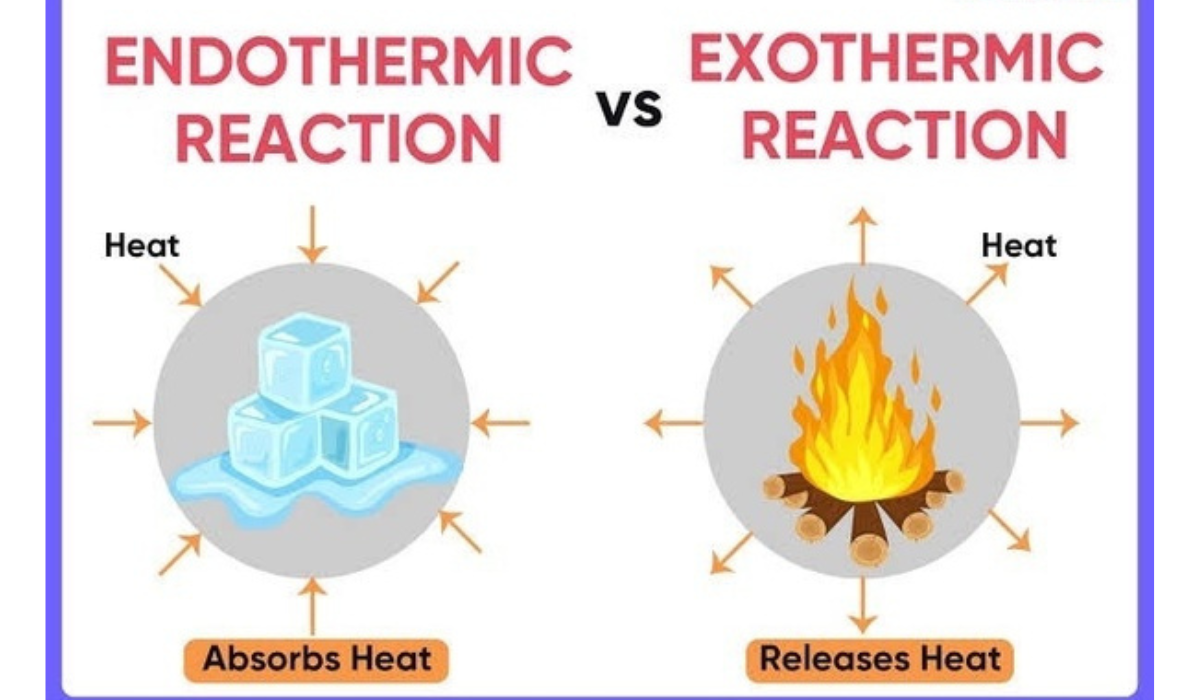
Comparing Endothermic and Exothermic Reactions
Understanding the differences between endothermic and exothermic reactions is crucial in chemistry. While both types involve energy changes during chemical processes, they behave oppositely.
Energy Absorption vs. Release
Endothermic Reactions absorb heat energy from their surroundings. A classic example is the melting of ice to form liquid water. Here, energy is required to break the bonds, leading to a cooling effect as heat energy is absorbed. Endothermic processes are essential in natural phenomena, such as photosynthesis, where plants absorb sunlight to convert carbon dioxide and water into glucose.
In contrast, Exothermic Reactions release energy in the form of heat. Combustion of fuels, like burning wood or gasoline, exemplifies this category; the reaction generates heat and light, indicating energy release. The formation of chemical bonds in exothermic reactions results in lower energy products compared to reactants.
Key Differences
| Feature | Endothermic Reactions | Exothermic Reactions |
|---|
| Energy Change | Absorbs energy | Releases energy |
| Temperature Effect | Causes surroundings to cool down | Causes surroundings to heat up |
| Examples | Melting ice, photosynthesis | Combustion, respiration |
Recognizing these fundamental differences is vital for grasping energy transformations in various chemical processes.
Energy Level Diagram of an Endothermic Reaction
An energy level diagram is a valuable tool for visualizing the energy changes in an endothermic reaction. In this type of reaction, the system absorbs heat from its surroundings.
Understanding the Diagram
1. Reactants and Products: The diagram typically features two horizontal lines representing the energy levels of the reactants and products. In an endothermic reaction, the energy level of the products is higher than that of the reactants, reflecting energy absorption.
2. Energy Barrier: The gap between the reactants and products shows the energy barrier that must be overcome, known as activation energy. This energy input facilitates the breaking of bonds in the reactants.
3. Energy Transformation: As the reaction proceeds, thermal energy is absorbed, leading to a transformation of chemical energy into potential energy. This is crucial in processes like photosynthesis, where plants absorb light energy.
4. Visual Representation: The simple energy level diagram helps illustrate this process. The upward slope towards the higher energy level symbolizes the endothermic nature, while the downward slope, if present, would illustrate an exothermic process.
In summary, energy level diagrams provide a clear visual representation of how energy is absorbed in endothermic reactions, enhancing our understanding of energy transformations in various chemical processes.
Conclusion
In summary, endothermic reactions are essential processes characterized by their ability to absorb heat energy, impacting various fields from refrigeration to biological functions. By understanding the principles of endothermic processes, such as the melting of ice and the evaporation of water, we see their importance in everyday life. These reactions not only demonstrate fundamental concepts of energy transformation and chemical changes but also play a critical role in innovative applications like chemical cold packs.
As we explore further, consider how endothermic and exothermic reactions complement each other in our world, especially in energy dynamics and temperature control. The intricate balance between these reactions contributes significantly to advancements in chemistry and related industries. We encourage you to dive deeper into this fascinating subject, examining how these reactions shape our environment and influence various processes. Your journey through the realm of endothermic reactions and their implications has just begun!




Post Comment