Chloroform Explained: Chemical Formula, Properties, and Reactions
Chloroform is a fascinating chemical compound that has captivated scientists and enthusiasts for decades. From its role in early anesthetics to its applications in modern chemistry, chloroform serves as a crucial piece of the chemical puzzle.
If you’re a chemistry enthusiast eager to understand the ins and outs of this compound, you’re in the right place. This blog will guide you through every aspect of chloroform—its chemical formula, properties, reactions, and significance—step by step.
By the end, you’ll have a clear understanding of why chloroform is so essential in the field of chemistry.
A Glimpse Into Its History
Chloroform was first discovered in 1831 independently by three scientists—Samuel Guthrie, Justus von Liebig, and Eugène Soubeiran. It became widely known after being employed as an anesthetic by James Young Simpson in the mid-19th century. However, due to safety concerns related to its toxic and carcinogenic nature, its anesthetic use has been phased out over time.
Even though chloroform is no longer used in medicine, its importance in the chemical world remains intact, particularly for its solvent properties and its role in organic synthesis.
What Is Chloroform?
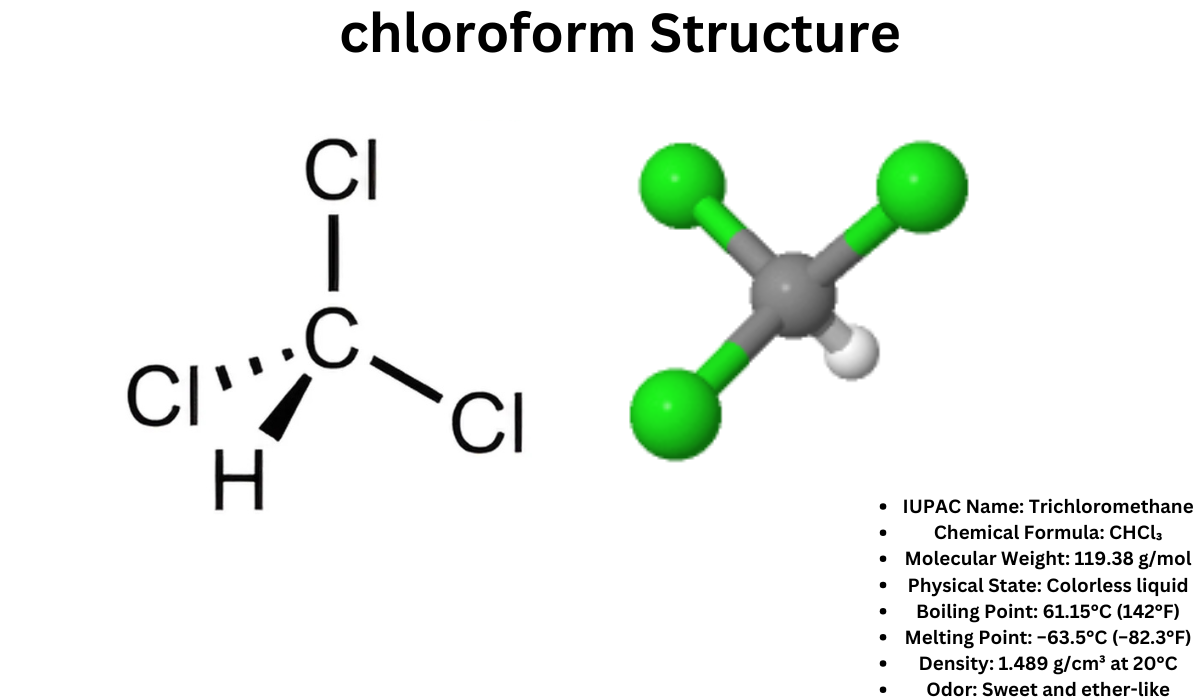
Chloroform, also known as trichloromethane, is an organic compound with the chemical formula CHCl₃. It is a colorless, sweet-smelling liquid that is slightly soluble in water. Widely used as a reagent and solvent in laboratories, chloroform has unique properties that make it a critical substance in various industries.
Chemical Structure and Properties
- IUPAC Name: Trichloromethane
- Chemical Formula: CHCl₃
- Molecular Weight: 119.38 g/mol
- Physical State: Colorless liquid
- Boiling Point: 61.15°C (142°F)
- Melting Point: −63.5°C (−82.3°F)
- Density: 1.489 g/cm³ at 20°C
- Odor: Sweet and ether-like
- Solubility: Slightly soluble in water (8 g/L at 20°C), highly soluble in organic solvents like alcohol and ether.
Chemical Properties
- Stability: It is relatively stable but decomposes slowly in the presence of light and air to form phosgene (COCl₂), a toxic gas.
- Polarity: Due to the presence of three electronegative chlorine atoms, It is polar but less so than water.
- Reactivity: It undergoes reactions typical of halogenated hydrocarbons, including substitution and elimination reactions.
How is Chloroform Produced?
Its production occurs at both laboratory and industrial scales. The production methods are as fascinating as the compound, relying on fundamental chemistry principles.
1. Industrial Production
Industrially, It is mainly produced via the chlorination of methane. Methane (CH₄) reacts with chlorine gas (Cl₂) to form a series of chlorinated products, including chloroform (CHCl₃). The reaction is typically carried out under controlled conditions with heat and UV light to drive desired results.
Reaction Sequence
CH₄ + Cl₂ → CH₃Cl + HCl (methyl chloride)
CH₃Cl + Cl₂ → CH₂Cl₂ + HCl (methylene chloride)
CH₂Cl₂ + Cl₂ → CHCl₃ + HCl (chloroform)
CHCl₃ + Cl₂ → CCl₄ + HCl (carbon tetrachloride)
The process can be optimized by controlling the reaction conditions to yield predominantly chloroform.
2. Laboratory Synthesis
On a smaller scale, chloroform can be synthesized in the lab using the haloform reaction. This method involves the reaction of ethanol or acetone with bleach (sodium hypochlorite, NaOCl).
Reaction Steps:
- Sodium hypochlorite reacts with acetone to form trichloromethyl intermediates.
- The intermediates are hydrolyzed to yield chloroform.
This simple yet effective method allows chemists to prepare chloroform in limited quantities for experimental use.
Reactions of Chloroform
Here’s where the chemistry gets exciting! It participates in various reactions that highlight its versatility in chemical processes.
1. Formation Reaction (Laboratory Synthesis)
Chloroform can be synthesized in the lab through the chlorination of methane.
Reaction:
CH₄ + 3Cl₂ → CHCl₃ + 3HCl
This reaction involves using chlorine gas to replace hydrogen atoms in methane step-by-step until chloroform is formed.
2. Reaction with Hydroxide (Base Hydrolysis)
Chloroform reacts with strong bases such as sodium hydroxide to form sodium formate (HCOONa).
Reaction:
CHCl₃ + NaOH → CO + NaCl + H₂O
This reaction highlights chloroform’s ability to release carbon monoxide under basic conditions.
3. Oxidation to Phosgene
When exposed to UV light or heat, chloroform undergoes oxidation to form phosgene, a toxic gas.
Reaction:
2CHCl₃ + O₂ → 2COCl₂ + 2HCl
Due to the dangers of phosgene, chloroform should always be appropriately stored in the absence of light.
4. Usage in Organic Synthesis
Chloroform is widely used in organic chemistry to produce dichlorocarbene (CCl₂), a highly reactive intermediate for further synthesis.
Reaction:
CHCl₃ + Base (e.g., KOH) → :CCl₂ + KCl
This reaction is utilized to create cyclopropane derivatives and other vital organic compounds.
Applications of Chloroform in Modern Chemistry
Chloroform remains a versatile and valuable chemical in both academic and industrial chemistry. Here are some of its most common uses:
1. Solvent
Chloroform is highly effective in dissolving nonpolar compounds, making it a preferred solvent for:
- Organic synthesis
- Extraction of natural products
- NMR Spectroscopy (in deuterated form, CDCl₃)
2. Reagent in Synthesis
Its role in generating dichlorocarbene (CCl₂) showcases chloroform’s importance in organic reactions.
3. Industrial Applications
Chloroform is employed industrially in the production of:
- Refrigerants (such as HCFC-22)
- PTFE (polytetrafluoroethylene), commonly known as Teflon
4. Medicine (Historical)
Although phased out, chloroform was used as an inhalation anesthetic during surgeries in the 19th and early 20th centuries.
Uses of Chloroform
- Historical Use as an Anesthetic: In the 19th and early 20th centuries, chloroform was used as an inhaled anesthetic during surgeries. However, its use was discontinued due to potential liver damage and the availability of safer alternatives.
- Industrial Applications:Solvent: Used to extract and purify antibiotics, alkaloids, and vitamins.
- Intermediate: Plays a role in the production of refrigerants like chlorodifluoromethane (R-22).
- Research and Laboratory Use: Commonly used as a chemical and biochemical research solvent.
- Used in DNA and RNA extraction protocols for its ability to denature proteins and separate nucleic acids.
- Pesticides and Herbicides: These are used as intermediates in synthesizing various agrochemicals.
- Deuterated Chloroform: Deuterated chloroform (CDCl₃) is widely used as a solvent in NMR spectroscopy due to its low reactivity and ability to dissolve a wide range of compounds.
- Fire Extinguishing: Chloroform was previously used in fire extinguishers as a component of carbon tetrachloride-based formulations.
Health and Environmental Concerns
- Toxicity: Chloroform can depress the central nervous system (CNS) and may cause dizziness, fatigue, and nausea upon exposure. Chronic exposure may damage the liver and kidneys.
- It is classified as a potential carcinogen.
- Environmental Impact: Chloroform is persistent in the environment and contributes to ozone depletion upon decomposition.
- Handling Precautions: To prevent the formation of phosgene, chloroform should be handled in well-ventilated areas, away from direct sunlight and heat.
- Proper protective equipment, such as gloves and goggles, is essential.
- when it reaches 300 ppb in water or 0.25 ppm in the air, it will damage the kidney or liver.
Safety Measures
- Storage: Store chloroform in amber bottles to block light exposure and prevent degradation.
- Disposal: Must be disposed of as hazardous waste following local regulations.
- Emergency Protocols: In case of accidental ingestion or inhalation, seek immediate medical attention. Avoid inducing vomiting.
Conclusion
Chloroform is a fascinating compound with diverse applications across science, industry, and medicine. While its use as an anesthetic is now a relic of the past, its role in modern chemistry and biochemistry remains significant. Proper handling and awareness of its health risks are essential to leveraging its benefits safely and effectively.
FAQs About Chloroform
Q1: What is the molecular formula of chloroform?
The molecular formula of chloroform is CHCl₃, and it consists of one carbon atom, one hydrogen atom, and three chlorine atoms.
Q2: What are the primary uses of chloroform today?
Chloroform is primarily used as a solvent in laboratories, a reagent in organic synthesis, and a precursor for refrigerant production (e.g., HCFC-22).
Q3: Is chloroform safe for humans?
No, chloroform is toxic and can cause dizziness, nausea, liver damage, and kidney damage upon prolonged exposure. It is also a potential carcinogen.
Q4: Why was chloroform discontinued as an anesthetic?
Chloroform was discontinued due to severe side effects like cardiac arrhythmias, respiratory depression, and liver toxicity, along with the development of safer alternatives.
Q5: How is chloroform made industrially?
Chloroform is synthesized by chlorinating methane or methyl chloride using heat and light.
Q6: Can chloroform be found naturally?
Yes, marine algae and other biological processes produce small amounts of chloroform.
Q7: What are the environmental effects of chloroform?
Chloroform can contaminate water and soil and contribute to the formation of toxic byproducts like trihalomethanes during water disinfection.
Q8: Does chloroform degrade over time?
Yes, chloroform can degrade in the presence of air and light, producing toxic compounds like phosgene (COCl₂). It is often stabilized with ethanol to prevent this.
Q9: What precautions should be taken when handling chloroform?
Use chloroform in well-ventilated areas, wear protective gloves and goggles, and store it in airtight containers away from light and heat.
Q10: Is chloroform flammable?
Chloroform is not flammable under normal conditions, but it can form explosive mixtures with certain chemicals, such as strong bases or reactive metals.



Post Comment