The pH of Distilled Water: A Comprehensive Guide
Introduction
Distilled water is often perceived as the purest form of water, free from contaminants, minerals, and impurities. However, its pH—a measure of acidity or alkalinity—is a topic of curiosity and occasional confusion. While pure water is theoretically neutral (pH 7), real-world factors can shift this value. This article explores the pH of distilled water, the science behind it, its practical implications, and how it compares to other types of water.
What Is Distilled Water?
Distilled water is produced through a purification process called distillation. This involves boiling water to create steam, which is then cooled and condensed back into liquid form. The process removes impurities, including minerals, salts, and organic compounds, leaving behind nearly pure H₂O.
Key characteristics of distilled water:
- Purity: Contains no dissolved ions or minerals.
- Conductivity: Poor electrical conductor due to lack of ions.
- Taste: Flat or bland compared to mineral-rich water.
Distillation is commonly used in laboratories, medical facilities, and industries where water purity is critical.
The pH of Distilled Water: Theory vs. Reality
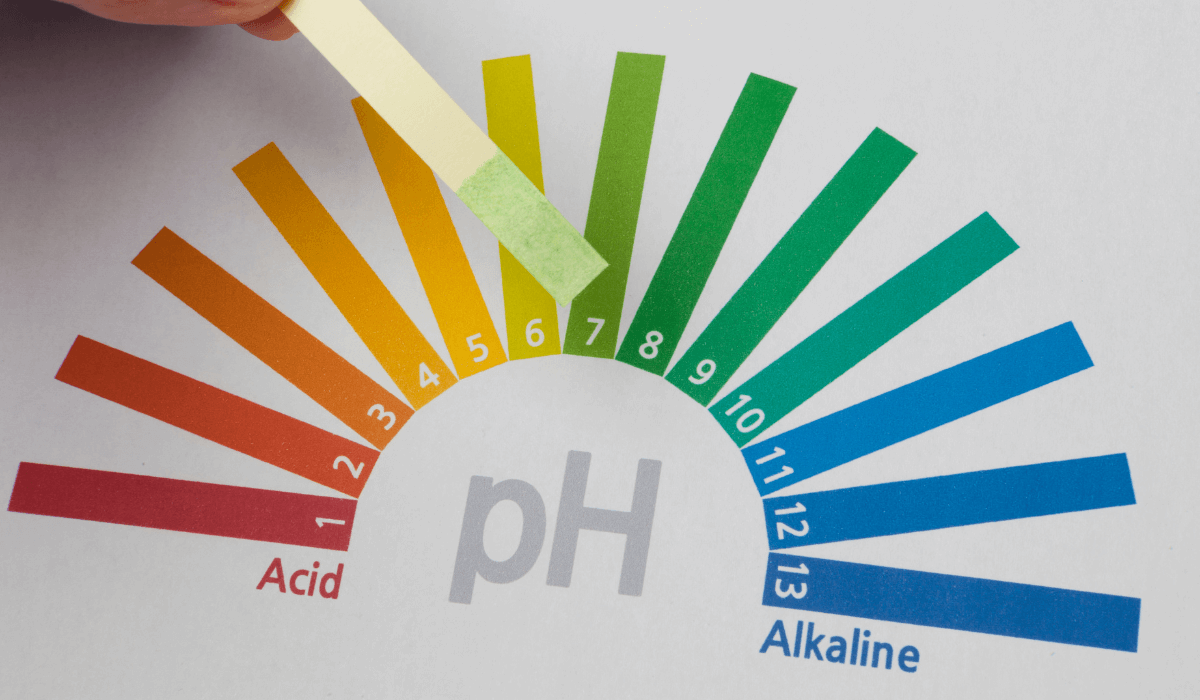
The pH scale ranges from 0 (highly acidic) to 14 (highly alkaline), with 7 being neutral. Pure water, in theory, has a pH of 7 at 25°C because the concentration of hydrogen ions (H⁺) equals hydroxide ions (OH⁻). However, distilled water’s pH in practice often deviates due to environmental interactions.
Why Distilled Water Isn’t Always pH 7
1. Absorption of Carbon Dioxide (CO₂):
When exposed to air, distilled water absorbs CO₂, which reacts with H₂O to form carbonic acid (H₂CO₃). This acid dissociates into bicarbonate (HCO₃⁻) and hydrogen ions (H⁺), lowering the pH. CO₂ + H₂O → H₂CO₃ → H⁺ + HCO₃⁻ This reaction can reduce the pH of freshly distilled water to 5.6–6.5, making it slightly acidic.
2. Temperature Effects:
pH is temperature-dependent. As temperature rises, water self-ionizes more, increasing H⁺ and OH⁻ concentrations. For example, at 100°C, pure water has a pH of 6.14, but it remains neutral since H⁺ and OH⁻ concentrations stay equal.
3. Storage Conditions:
Storing distilled water in plastic containers may introduce organic compounds, while glass containers minimize contamination. Prolonged exposure to air accelerates CO₂ absorption.
Measuring the pH of Distilled Water
Accurate pH measurement requires precision due to distilled water’s low ionic content:
- Calibrate pH Meters: Use buffer solutions (pH 4, 7, and 10) to ensure accuracy.
- Avoid Contamination: Rinse electrodes with distilled water before use.
- Measure Quickly: Test immediately after collection to minimize CO₂ exposure.
Note: pH strips may be less reliable for distilled water due to its low conductivity.
Comparing Distilled Water to Other Water Types
- Tap Water:
- Contains minerals (e.g., calcium, magnesium) and additives (e.g., chlorine).
- Typically pH 6.5–8.5, varying by location.
- Deionized (DI) Water:
- Purified via ion exchange resins, removing charged impurities.
- Similar pH instability as distilled water due to CO₂ absorption.
- Spring/Mineral Water:
- Naturally alkaline (pH 7.5–8.5) due to dissolved minerals like bicarbonate.
- Rainwater:
- Slightly acidic (pH ~5.6) from atmospheric CO₂, similar to distilled water.
Applications Where pH Matters
- Laboratory Use:
Distilled water’s purity makes it ideal for experiments, but its variable pH requires calibration in sensitive procedures (e.g., cell cultures). - Medical Equipment:
Used in sterilizers and CPAP machines; low mineral content prevents scaling. - Automotive Batteries:
Low ion content prevents corrosion, though some manufacturers specify pH-adjusted water. - Aquariums:
Rarely used directly; mixed with tap water to stabilize pH for aquatic life.
Debunking Myths About Distilled Water
- Myth 1: “Distilled water leaches minerals from the body.”
Fact: While it lacks minerals, moderate consumption doesn’t harm health. - Myth 2: “All distilled water is acidic.”
Fact: Freshly distilled water approaches pH 7 but becomes acidic over time. - Myth 3: “Boiling water makes it distilled.”
Fact: Boiling kills microbes but doesn’t remove minerals; distillation requires condensation.
Enhancing Distilled Water’s pH Stability
To maintain neutrality:
- Store in airtight glass containers.
- Add a pinch of baking soda (sodium bicarbonate) to buffer pH.
- Use within 24 hours for critical applications.
Conclusion
Distilled water’s pH is a dynamic property influenced by environmental factors, primarily CO₂ absorption. While it starts close to neutral, its acidity increases over time, underscoring the importance of proper storage and handling. Understanding its behavior is essential for applications ranging from scientific research to everyday use. By demystifying its properties, users can harness distilled water’s benefits while mitigating its limitations.
Final Thought: Pure water is a concept—real-world conditions ensure that even distilled water is in a delicate balance with its surroundings. Whether in a lab or a household, respecting this balance unlocks its full potential.



Post Comment