Chemical Bonding: Understanding Ionic vs. Covalent Bonds
Chemistry is all around us, and understanding how atoms interact is crucial to grasping the fundamentals of the subject. One of the key concepts in chemistry is bonding, specifically ionic and covalent bonds. In this blog, we’ll dive deep into these two types of Chemical Bonding, their formation, and how they relate to the stability of atoms.
The Octet Rule: A Foundation for Stability
Atoms are constantly seeking stability. The outer shell of many atoms, with hydrogen being a notable exception, typically holds eight electrons. When an atom has eight electrons in its outer shell, it is considered chemically stable. This principle is known as the Octet Rule. Atoms want to achieve this stable configuration, and they can do so by giving up, accepting, or sharing electrons with other atoms.
Covalent Bonds: Sharing Electrons
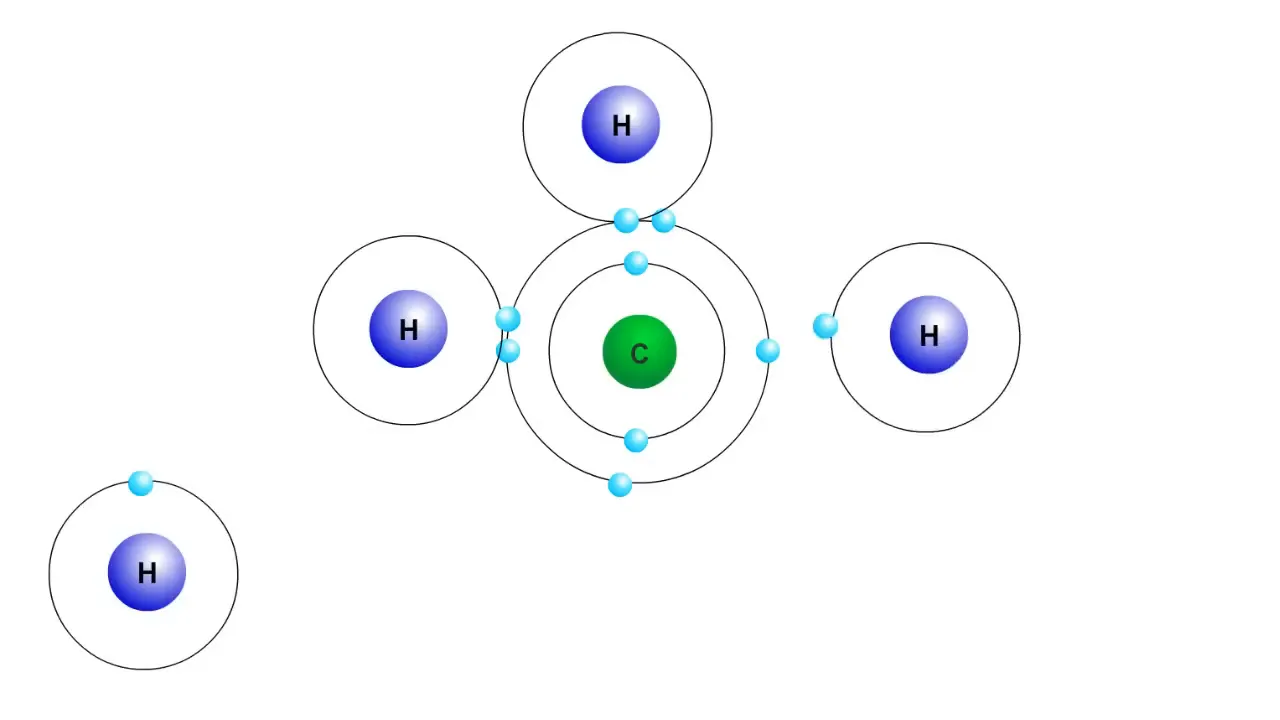
A covalent bond forms when atoms share electrons to satisfy the Octet Rule. For instance, a carbon atom has four electrons in its outer shell but becomes much more stable with eight. To gain these additional four electrons, carbon can share its electrons with other atoms. When a carbon atom bonds with four hydrogen atoms, they share their electrons, allowing carbon to achieve a complete outer shell.
In this configuration, each hydrogen atom also achieves two electrons in its outer shell, which is the stable configuration for their first electron shell. This sharing of electrons is what characterizes covalent bonding, leading to the formation of molecules.
Ionic Bonds: Attraction of Opposites
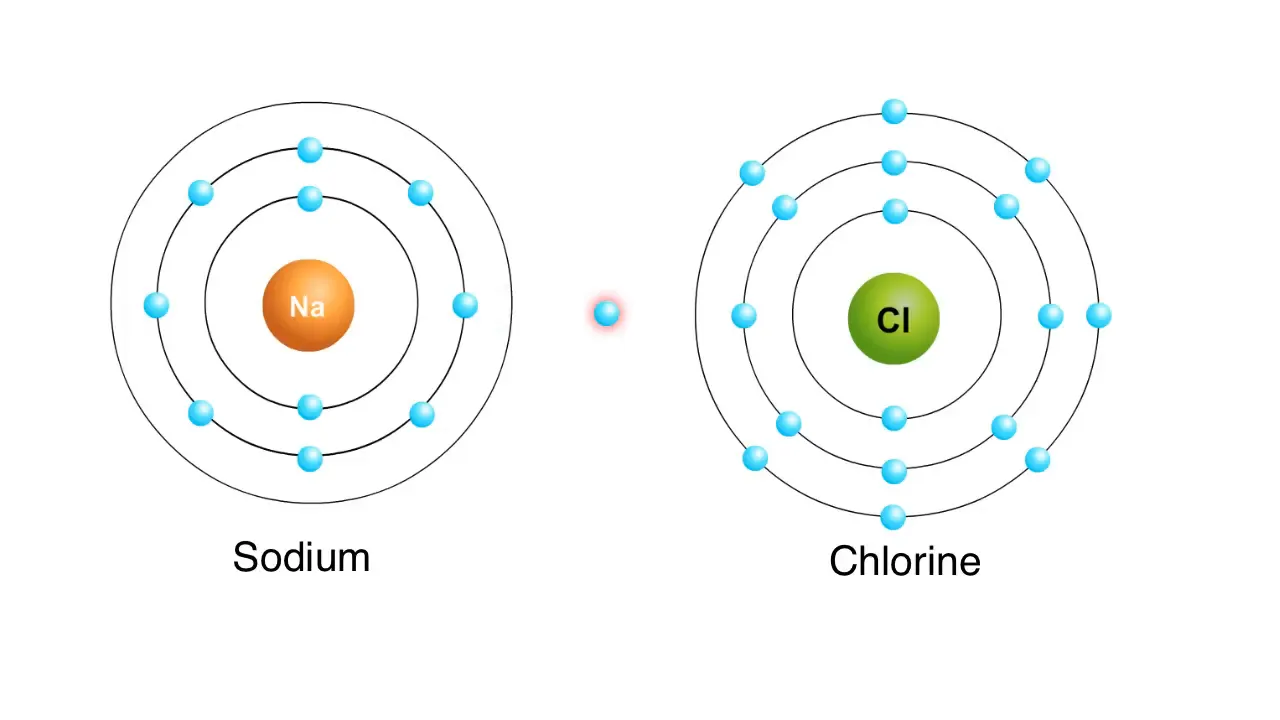
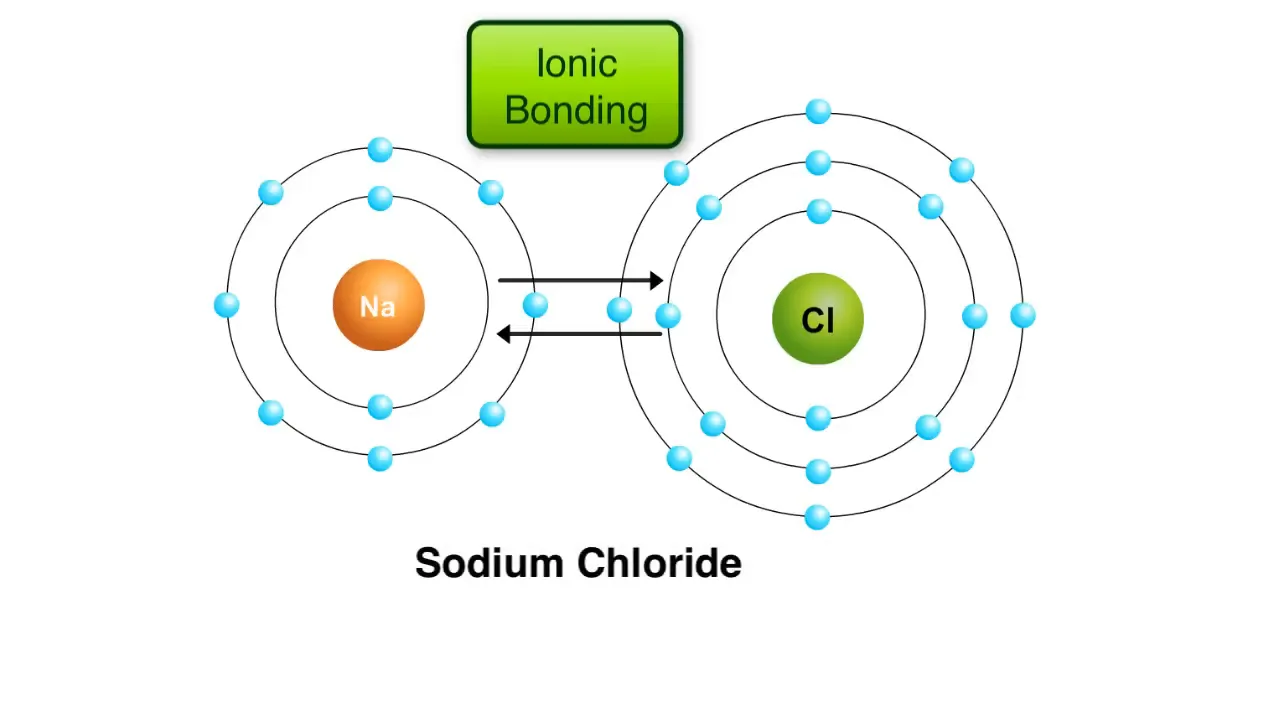
On the other hand, ionic bonds form when two atoms are held together by the attraction between opposite charges. A classic example of this is the reaction between sodium and chlorine atoms. Sodium has one electron in its outer shell and tends to give it up easily. Once sodium loses this electron, its outer shell is filled with eight electrons, giving it a slight positive charge.
Chlorine, with seven electrons in its outer shell, tends to gain an electron. When chlorine accepts an electron from sodium, it becomes negatively charged. The resulting charged sodium and chlorine atoms are known as ions, and these oppositely charged ions attract each other, forming an ionic bond. This reaction leads to the creation of sodium chloride, commonly known as table salt.
Comparing Ionic and Covalent Bonds
Both ionic and covalent bonds play crucial roles in the formation of compounds, but they do so in fundamentally different ways. Let’s break down some key differences:
- Electron Transfer vs. Sharing: Ionic bonds involve the transfer of electrons from one atom to another, while covalent bonds involve the sharing of electrons.
- Formation of Ions: Ionic bonds result in the formation of charged ions, whereas covalent bonds do not create charged particles.
- Strength: Ionic bonds are generally stronger due to the electrostatic attraction between oppositely charged ions, while covalent bonds can vary in strength depending on the number of shared electron pairs.
Real-World Examples of Ionic and Covalent Bonds
Understanding these bonds is essential because they dictate the properties of the substances we encounter daily. For example, sodium chloride (table salt) is a classic ionic compound. Its high melting point and ability to conduct electricity in solution are due to the strong ionic bonds present in its lattice structure.
In contrast, water (H2O) is a covalent compound. The sharing of electrons between oxygen and hydrogen atoms allows water to have unique properties such as a high boiling point relative to its molecular weight, which is a result of hydrogen bonding, a special type of intermolecular force related to covalent bonding.
Conclusion: The Importance of Understanding Bonds
In summary, both ionic and covalent bonds are fundamental concepts in chemistry that explain how atoms interact to form stable compounds. The Octet Rule guides these interactions, whether atoms choose to share electrons in covalent bonds or transfer them in ionic bonds. Understanding these principles not only helps us comprehend chemical reactions but also lays the groundwork for exploring more complex topics in chemistry and beyond.
By recognizing the differences between ionic and covalent bonds, we can better appreciate the diverse array of substances that make up our world and how they contribute to the myriad of processes we encounter in our daily lives.













Post Comment