A Beginner’s Guide to the Periodic Table: Fun Facts and Uses
The periodic table is one of the most powerful tools in chemistry, organizing all known elements in a way that reveals trends and relationships between them. Understanding the periodic table is a key foundation for anyone interested in chemistry. This guide will introduce you to the periodic table, covering everything from the basics to fun facts, and explain the terms and concepts that make this table so essential in science.
After studying this article, a beginner will have a clear understanding of the core concepts of the periodic table, from its structure to the trends that govern it. With A Beginner’s Guide to the Periodic Table, you now have the foundation to explore the fascinating world of chemistry.
1. What is the Periodic Table?
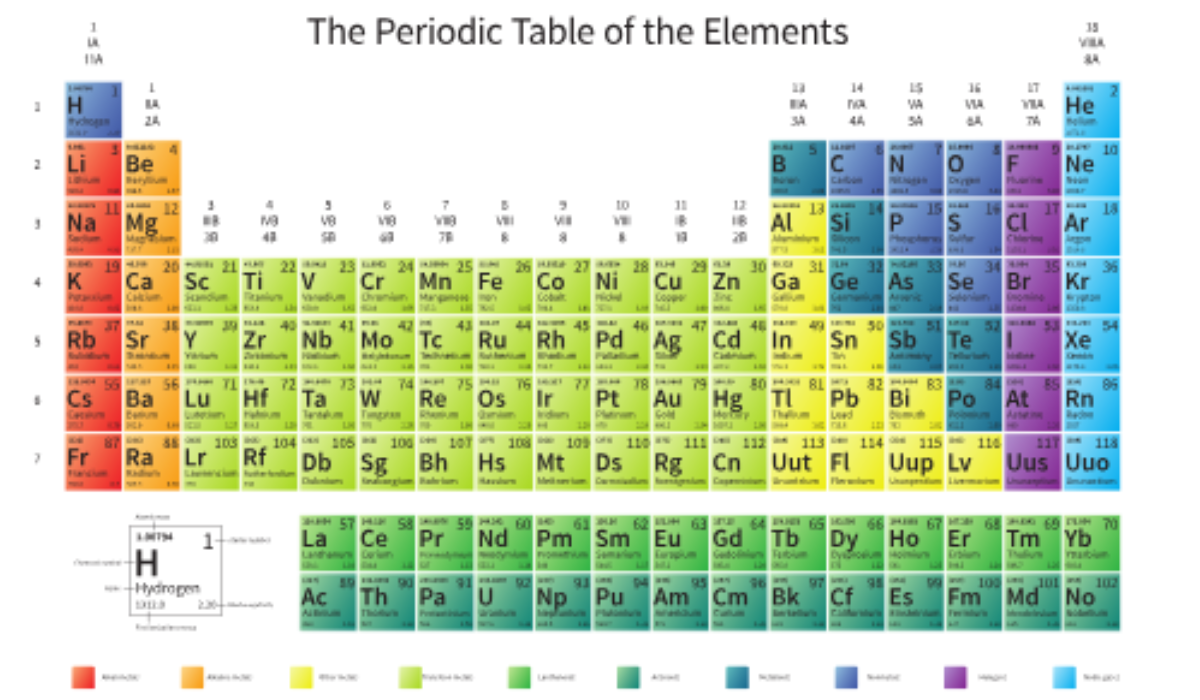
The Periodic Table of Elements is a tabular arrangement of chemical elements, organized by increasing atomic number. Each element is placed in a specific position based on its properties, such as atomic mass, electron configuration, and chemical behavior. The periodic table is divided into rows (called periods) and columns (called groups or families), making it easy to see patterns and relationships between elements.
2. The History of the Periodic Table
The periodic table was first organized by Dmitri Mendeleev, a Russian chemist, in 1869. He noticed that when elements were arranged in order of increasing atomic mass, elements with similar chemical properties occurred at regular intervals. This became the foundation for the Periodic Law, which states that the properties of elements recur periodically when arranged by atomic number.
3. What is Atomic Number?
Each element in the periodic table is identified by its atomic number, which is the number of protons found in its nucleus. The atomic number determines the identity of the element. For example, hydrogen has an atomic number of 1 because it has one proton, while helium has an atomic number of 2 because it has two protons. The atomic number is what sets elements apart and helps in their arrangement on the table.
4. Understanding Electron Configuration
Electron configuration refers to the arrangement of electrons around the nucleus of an atom. Electrons fill orbitals in order of increasing energy levels, with each element having a specific electron configuration. The periodic table groups elements based on their electron configuration. For instance, elements in the same column (group) often have similar electron configurations and, therefore, exhibit similar chemical properties.
5. Periodic Trends in the Table
The periodic table reveals periodic trends, which are predictable patterns that occur as you move across periods (rows) or down groups (columns). Some common periodic trends include:
- Ionization Energy: The energy required to remove an electron from an atom. As you move across a period, ionization energy increases because atoms become smaller and electrons are held more tightly.
- Atomic Radius: The size of an atom. As you move across a period, the atomic radius decreases, but as you move down a group, it increases because additional electron shells are added.
- Electron Affinity: The energy change that occurs when an electron is added to an atom. Elements in the upper-right corner of the table, such as halogens, have high electron affinity because they are eager to gain electrons to complete their outer shells.
6. Valence Electrons: The Key to Chemical Bonding
Valence electrons are the electrons in the outermost shell of an atom, and they play a critical role in chemical reactions. These electrons are involved in bonding with other elements to form molecules. For example, elements in Group 1 (alkali metals) have one valence electron and tend to lose it easily, forming positive ions. On the other hand, elements in Group 17 (halogens) have seven valence electrons and are likely to gain one more to complete their outer shell.
7. The Importance of Groups in the Periodic Table
The periodic table is organized into 18 groups, or columns, that categorize elements with similar properties. Some well-known groups include:
- Alkali Metals (Group 1): These are soft, highly reactive metals like lithium, sodium, and potassium.
- Alkaline Earth Metals (Group 2): Elements like magnesium and calcium, which are slightly less reactive than alkali metals but still form strong bonds.
- Transition Metals (Groups 3–12): These include familiar elements like iron, copper, and gold. They have unique properties and are often used in construction and technology.
- Noble Gases (Group 18): The inert gases like helium, neon, and argon. These elements are chemically stable because their valence electron shells are full.
8. What Are Periods and How Do They Work?
The periods are the horizontal rows of the periodic table. As you move from left to right across a period, the atomic number increases, and elements show a progression in their chemical properties. Each new period starts with an element that has one electron in its outer shell and ends with an element that has a full outer shell, making it chemically stable.
9. The Periodic Table Blocks (s, p, d, f blocks)
The elements of the periodic table are further categorized into four blocks based on their electron configuration:
- s-block: Includes Group 1 and Group 2 elements (except helium), which have their outermost electron in an s orbital.
- p-block: Includes Groups 13 to 18 elements, characterized by electrons filling p orbitals.
- d-block: The transition metals (Groups 3 to 12), where electrons fill d orbitals.
- f-block: Lanthanides and actinides, which are often placed below the main table and fill f orbitals.
10. Fun Facts About the Periodic Table
- Noble Gases are the only group of elements that do not form compounds easily because their outer electron shells are full.
- The periodic table contains 118 known elements, with 116 officially named.
- Uranium, with an atomic number of 92, is the heaviest naturally occurring element.
- The symbol for gold (Au) comes from the Latin word “aurum,” meaning “shining dawn.”
11. Applications of the Periodic Table in Real Life
The periodic table is not just a classroom tool; it has numerous practical applications in daily life and science. Some examples include:
- Medicine: Elements like iodine and phosphorus are crucial for biological processes and medical imaging.
- Technology: Silicon, a metalloid from the periodic table, is a key component in the manufacture of semiconductors and computers.
- Energy: Elements like uranium and plutonium are used in nuclear reactors to generate energy.
- Environmental Science: Understanding the behavior of elements in the environment can help with pollution control and water purification.
12. Conclusion
The periodic table is more than just a list of elements—it’s a roadmap to understanding the building blocks of matter. Whether you’re studying chemistry or just curious about how the world works, the periodic table offers a wealth of knowledge. From the atomic number to the trends in electron configurations, mastering the periodic table opens the door to deeper insights into both science and everyday life. Embrace the patterns, explore the facts, and see how this essential tool connects the elements of the universe!
Frequently Asked Questions (FAQs) about Periodic Table
1. Why is the Periodic Table Arranged by Atomic Number Instead of Atomic Mass?
Originally, elements in the periodic table were arranged by their atomic mass, but this method led to inconsistencies. For example, iodine and tellurium were out of order when arranged by atomic mass. The modern periodic table is organized by atomic number (the number of protons in the nucleus), which resolves these inconsistencies. This arrangement also reflects the periodic law, where properties of elements repeat at regular intervals when ordered by atomic number.
2. What Are Isotopes and How Do They Relate to the Periodic Table?
Isotopes are atoms of the same element that have the same number of protons but different numbers of neutrons. This means they have the same atomic number but different atomic masses. The periodic table shows the atomic number (protons) but typically lists the average atomic mass, which accounts for the isotopes’ varying abundances. For example, carbon has two stable isotopes, carbon-12 and carbon-14.
3. Why Do Some Elements Have Different Versions of Their Symbols?
Some elements have symbols derived from their Latin names, which can seem confusing. For example, the symbol for gold is “Au,” from the Latin “aurum,” and iron has the symbol “Fe,” from the Latin “ferrum.” This is a historical tradition from when many elements were first discovered and named in ancient times, long before the international system of chemical symbols was standardized.
4. What Are Lanthanides and Actinides, and Why Are They Separate from the Main Table?
The lanthanides and actinides are two rows of elements placed separately at the bottom of the periodic table to keep the table compact. These elements are part of the f-block and have special electron configurations. The lanthanides (elements 57 to 71) are known for their high magnetic properties, while the actinides (elements 89 to 103) include radioactive elements like uranium and plutonium. They are separated to avoid making the main table unnecessarily wide and complex.
5. How Are New Elements Added to the Periodic Table?
New elements are created in laboratories through nuclear reactions, often by colliding atoms at very high speeds. These elements are typically unstable and decay quickly into other elements. Once a new element is discovered, it must undergo rigorous testing and verification before it is officially added to the periodic table. The element’s discovery is usually acknowledged by a team of scientists, and the new element is given a name and symbol by the International Union of Pure and Applied Chemistry (IUPAC). The most recent additions, elements 113 (Nihonium), 114 (Flerovium), 115 (Moscovium), 116 (Livermorium), 117 (Tennessine), and 118 (Oganesson), were officially recognized in the last decade.




Post Comment